We have developed a novel correlative imaging and analysis technique that combines optical cryo‑fluorescence imaging with high resolution morphological (cryo-SEM), spectroscopic (cryo-EDS) and electron density (BSE) characterization in the scanning electron microscope.
For the correlative imaging, live specimens of foraminifera, unicellular marine protozoans with calcitic shells, were cultured in calcein-containing sea water. The specimens were prepared for cryo-SEM imaging by high pressure freezing and freeze fracture. Each foraminifer exposed in the fracture was first imaged in cryo-SEM at low magnification to obtain an overview image of the fracture surface, and then systematically scanned in SE and BSE mode at medium magnification. These images were stitched together to provide a detailed view of the cytoplasm contents. Specific areas of interest were further imaged at higher magnification. The vitrified specimens were then transferred in a cryo-Correlative Light Electron Microscopy (cryo-CLEM) stage to the confocal microscope, and fluorescence images were taken. Maximum intensity projections were used for alignment to the SEM images. Adjustments to the alignment were made using the shell outline and the intracellular symbionts as anchors in the SE mode collage and the fluorescence image (Fig. 1).
Using this correlative approach, we studied the foraminifer cell cytoplasm under close to physiological conditions, without dissolution of the shell, and analyzed the elemental compositions of different phases within their cellular context. We also observed Mg2+-rich dense mineral particles both in the cytosol and in sea water vacuoles (Fig. 2). We suggest these may be related to the mechanism of removal of excess Mg2+ from sea water. Imaging the interior of shelled organisms is difficult by traditional light microscopy due to scattering by the shell itself. The new method provides a high resolution view of the cytoplasm, symbionts, and transport vesicles where ions may be concentrated or removed. Ion-sensitive fluorescent dyes and cryo-EDS mapping are used to identify the ions. This technique may well have widespread applications to many other shelled single-cell mineralized organisms in addition to foraminifera, such as coccolithophorids, diatoms, radiolaria, ciliates and acantharia etc., as well as for documenting aspects of mineral transport pathways in multicellular organisms.
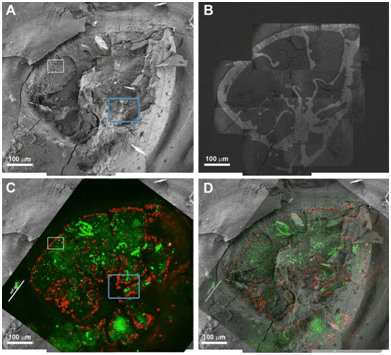
Figure 1: Correlative cryo-SEM/fluorescence microscopy. A) Low magnification cryo-SEM image in SE mode of an A. lessoni foraminifer; B) The same specimen imaged in BSE mode. The shell mineral is seen with high contrast. This image was obtained by stitching together tens of higher magnification images that were taken with partial overlap to facilitate reconstruction. C) Fluorescence image of the same specimen. Symbionts are red and calcein staining is green. D) Overlay of the fluorescence image on the electron microscope image. The shell and the symbionts are used for alignment.
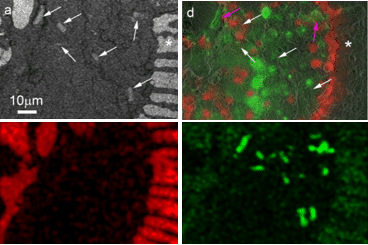
Figure 2: Mg rich particles in a specimen of A. lobifera. Correlative microscopy. Above: BSE micrograph and overlay of fluorescence on SE micrograph. The white arrows mark the position of the Mg rich particles, which are electron dense, but are only weakly fluorescent. Below: Cryo-EDS mapping, Ca map and Mg map: In the Ca map, the shell is clearly visible, in the Mg map the signal from the particles is very strong.

